

Laboratory accreditation to iso iec 17025. Section 8 - Management System Requirements: Options, Management System Documentation, Control of Management System Documentation, Control of Records, Risks and Opportunities, Improvements, Corrective Actions, Internal Audit, Management ReviewsĬanadian Association for Laboratory Accreditation Section 7 - Process Requirements: Review of requests, tenders and contracts, Method Selection, Sampling, Handling of Customer Items, Technical Records, Evaluation of Measurement Uncertainty, Validity of Results, Reporting, Complaints, Nonconforming work, Control of Data and Information Management (NEW) Section 6 - Resource Requirements: General, Personnel, Facility and Environment, Equipment, Metrological Traceability, External Products and Services Discussion in 'ISO 17025 - Calibration and Test Laboratories' started by RalphD, Feb 24, 2019. Section 5 - Structural Requirements: Legal Entity, Management, Range of Laboratory Activities (NEW), Conformity, Availability and Authority of Resources, Personnel and Integrity Section 4 - General Requirements: Impartiality and Confidentiality The new requirement sections are as follows: INTRODUCCIN 1.1 Distribucin Las copias impresas y digitales de este documento no estn controladas y los usuarios deben verificar que la revisin est vigente antes de su uso.
Iso 17025 2017 manua manual#
There are now 5 requirements sections instead of the 2 requirements sections (section 4 and 5) from the 2005 version of the standard. Cdigo: MC-SGC-001 MANUAL DE CALIDAD ISO/IEC 17025:2017 Versin: 3 Vigente desde: Pgina 1 de 52 1. The ISO 17025 standard was re-issued in 2017 as a replacement to the 2005 version. IEC: International Electrotechnical Commissionġ7025: Unique standard number assigned by ISO ISO: International Organization for Standardization
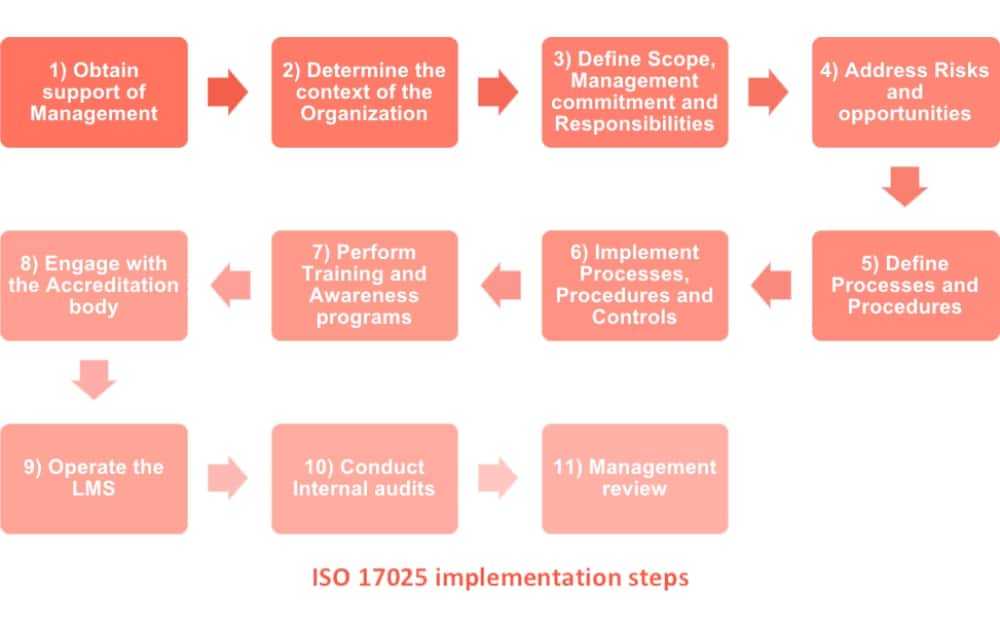
ISO/IEC 17025:2017 - General requirement for the competence of testing and calibration laboratories The laboratory needs a plan to address risks, however, there is no requirement for a formal risk management system or documented risk management process.Ĭontents of the ISO/IEC 17025:2017 International Standard The ISO/IEC 17025 standard can be obtained through ISO at the following link: This can be accomplished with policies and procedures if the lab prefers or by evidence of records such as training, internal audit, etc. For example, ISO/IEC 17025:2005 stated "the laboratory shall have policies and procedures to ensure protection of confidential information" and ISO/IEC 17025:2017 states "the laboratory shall ensure the protection of confidential information.". There is more latitude on how laboratories can implement the standard.

Risk based thinking applied in this version enables some reduction in prescriptive requirements and their replacement by performance based requirements. The ISO/IEC 17025 international standard was revised in 2017.Īddition of processes rather than procedures, risk based thinking and management, identifying opportunities in business, quality and customer satisfaction, measured performance, prevention of negative impacts, effective improvements, separated technical and Management System Records.


 0 kommentar(er)
0 kommentar(er)
